Lilac SolutionsTransforming lithium extraction to increase supply and decrease negative environmental impact
Facebook can be a great way to connect with friends, find local events, or solve the dramatically growing demand for lithium. Or at least that’s what David Snydacker used the popular social networking site for. The idea for his company, Lilac Solutions, was born out of a casual Facebook chat.
A PhD student in Christopher Wolverton's lab, professor of materials science and engineering, Snydacker had been conducting research on ways to power a vehicle with lithium batteries. Aware of this work, Snydacker’s friends sent him questions via Facebook. They wanted to know the environmental impact of lithium and whether or not there would be enough lithium to convert all cars to full battery power. After all, the mainstream success of the electric car relies on a hearty lithium supply.
To answer these questions, Snydacker dove into the world of lithium extraction. He discovered that while there is enough lithium on the planet, there should be a better way to access it.
The Problem
Most of the world’s lithium reserves are found in salt brines, located in South America, China, and the United States. The conventional process for extracting lithium from brines involves transferring the brine to small ponds where the water is evaporated. Not only are these brine ponds expensive to build and maintain, but the evaporation process takes 18 to 24 months. After the remaining lithium is removed from the pond, residual salt is collected and stored in massive, toxic salt piles. Even after all this money and effort, this process only recovers 50 percent of the lithium available in the brine and cannot access lower-quality lithium resources.
In my research to answer these questions, I uncovered mostly good news about the future of lithium, but I also uncovered a big opportunity. New extraction technologies are needed to ramp up supply., Lilac Solutions, chief technology officer
The Solution
Snydacker’s proposed solution uses ion exchange, an alternative process for extracting lithium that can boost lithium production and access new, lower-quality resources. Instead of using evaporation ponds, the process extracts lithium by using materials that can selectively absorb lithium from brine and then release it at a high concentration.
Snydacker partnered with colleagues in Christopher Wolverton’s research group to search for new ion exchange materials. Using Wolverton’s previously created Open Quantum Materials Database, Snydacker identified 13 potential new materials. The extensive database allows users to search materials by compositions. It also uses machine-learning that can learn chemistry to predict the possible existence of new compounds that have not yet been synthesized. Snydacker founded Lilac Solutions to develop these materials for ion exchange — materials that can extract lithium faster, at a higher concentration, and with a much smaller carbon footprint.
How It Works
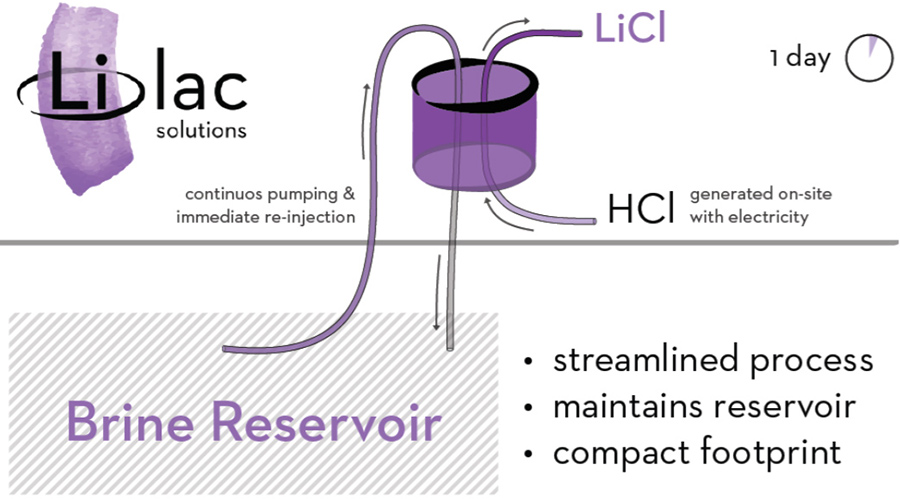
Lilac Solutions plans to pump brine directly from a brine reservoir into a tank filled with materials specialized for ion exchange. When the brine filters through the material, it releases lithium to be collected. The remaining salt waste will be drained directly back into the brine reservoir. This streamlined process bypasses the need for the brine to be transported to evaporation ponds and eliminates the toxic salt piles that result from the conventional process.
Benefits
- Recovers 80 percent of lithium as opposed to 50 percent
- Brine is pumped and then injected back into the reservoir to prevent depletion
- The conventional process takes two years; Lilac Solutions’ process takes one day
- Site is powered by generated, on-site electricity
- Process eliminates toxic, space-wasting evaporation ponds
- Process is safer and less toxic than the conventional process
Current Status
Two of the materials identified by the Open Quantum Materials Database were tested experimentally to validate the model. Lilac Solutions has plans to test the remaining 11 materials. Northwestern has filed provisional patent applications for Lilac Solutions’ materials and process. In 2016, Lilac Solutions won $15,000 and the Green Energy + Sustainbility Track at the 2016 Northwestern Venture Challenge.
In 2017, the startup was awarded a Phase 1 Small Business Innovation Research (SBIR) grant from the Department of Energy. The funding enabled Lilac to expand its portfolio of unique ion exchange materials for lithium extraction.
Lilac Solutions received recognition from Fast Company as a "World Changing Idea" in 2017, and in January 2018, the company announced the closing of its $800,000 seed financing round.
In October 2022, the U.S. Department of Energy awarded Lilac Solutions a $50 million grant to advance domestic lithium production, propelling the company to support U.S. leadership in the green-energy transition.
Updated November 2022